
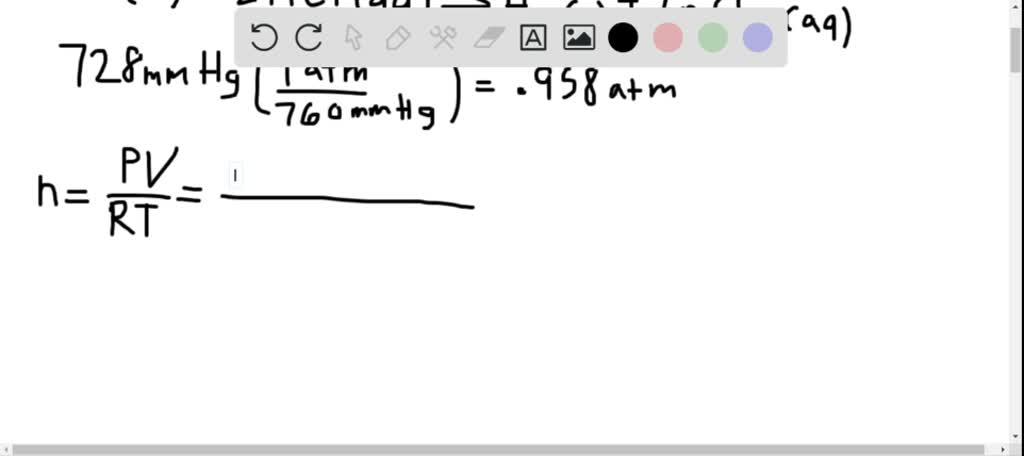
728 mmhg to atm full#
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. How many Atmospheres in a Millimeter Mercury One Millimeter Mercury is equal to 0. The pressure p in atmospheres (atm) is equal to the pressure p in millimeter mercury (0°c) (mmHg) times 0.00132, that conversion formula: p(atm) p(mmHg) × 0.00132. You can view more details on each measurement unit: mmHg or atm The SI derived unit for pressure is the pascal. 1 millimeter mercury (mmHg) is equal to 0.00132 atmospheres (atm). We assume you are converting between millimeter of mercury 0 C and atmosphere standard.
It is approximately equal to Earth's atmospheric pressure at sea level.Ĭonversion calculator for all types of measurement units. How many mmHg in 1 atm The answer is 759.9998769899. It is sometimes used as a reference pressure or standard pressure. What is the pressure when the volume of the gas is reduced to on tenth (0.10) of the original value at the same temperature A sample of air occupies 3.8 L when the pressure is 1.2 atm. The standard atmosphere (symbol: atm) is a unit of pressure defined as 101325 Pa (1.01325 bar). At 46 degrees C a sample of ammonia gas exerts a pressure of 5.3 atm. The torr is a non-SI unit of pressure, named after Evangelista Torricelli. Type in your own numbers in the form to convert the units Quick conversion chart of torr to atm.
728 mmhg to atm how to#
Use this page to learn how to convert between torrs and atmospheres. Note that rounding errors may occur, so always check the results. If 1 atm has to be converted to bar, $1atm\,=\,1.You can do the reverse unit conversion fromĪtm to torr, or enter any two units below: Enter two units to convert From: 1 pascal is equal to 0.0075006168270417 torr, or 9.8692326671601E-6 atm. Let’s see some interconversion from 1 atm. If the volume of a gas is 25.8 mL at 28.7☌ and 728 mmHg, what is its volume at 15.2☌ and 757 mmHg You should be able to do this calculation by reading the experimental write-up. Note: We know the interconversion between the units to do various calculation problems. Note: 1 atm 760 mm Hg where 760 is an exact number (not 2 sig.

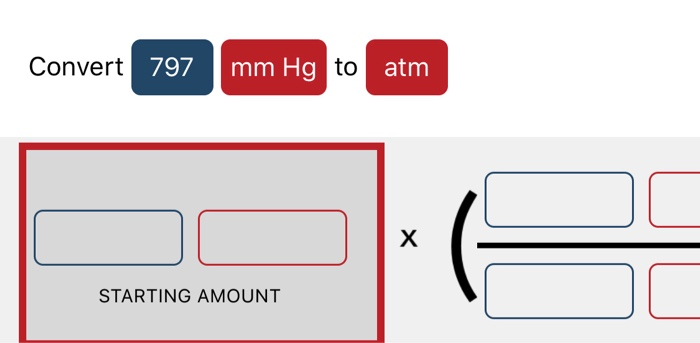
A tank contains a mixture of helium, neon. an aerosol can have a pressure of 1.75 atm what is this pressure in units of mm hg. What is the partial pressure of nitrogen. The partial pressures of oxygen and argon are 234 mmHg and 135 mmHg respectively. The formula to convert from mmHg to atm is: atm mmHg ÷ 760. So we can expresses the equation of pressure as, A mixture of nitrogen, oxygen and argon has a total pressure of 728 mmHg. Lets take a closer look at the conversion formula so that you can do these conversions yourself with a calculator or with an old-fashioned pencil and paper.

We know that pressure is the force exerted in a specific area and it is calculated by dividing the force applied by the area on which the force was applied. So before going into the solution part of the question, let’s discuss some concepts about pressure and its unit. In the question, it is asked that we have to convert the given value of unit in mm Hg to atmospheres of pressure.The question is simply about te unit conversion of values between the units of pressure, You can do it like this: 760'mmHg'1' ''Atm' .1'mmHg'1/760' ''Atm' .520'mmHg'1/(760)xx5200. We should be familiar with 1 atm value equal to how much value of mmHg. Calculate the final volume of the bubble if its initial volume was 2.1 mL.
4.52 A small bubble rises from the bottom of a lake, where the temperature and pressure are 4☌ and 3.0 atm, to the waters surface, where the temperature is 25☌ and the pressure is 0.95 atm. Hint: The approach for the question should be done by focusing on the unit conversion of the value from mmHg to atmospheres of pressure in atm. A) 0.095 atm B) 0.717 atm C) 3.26 atm D) 4.52 atm E) 34.2 atm D.


 0 kommentar(er)
0 kommentar(er)
